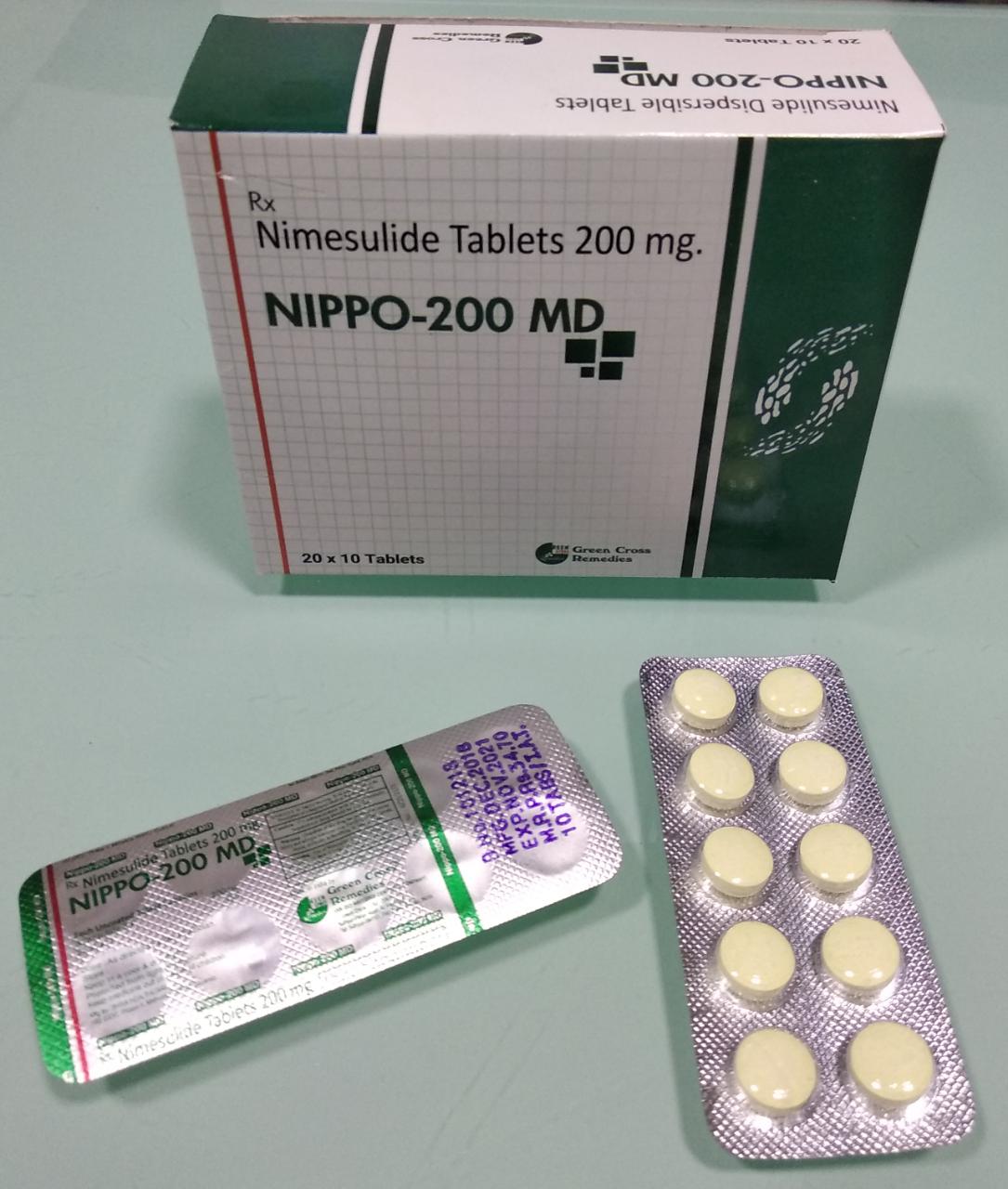
NIPPO 200 MD TABLET

NIPPO 200 MD TABLET
Nimesulide 200 mg Flavoured Mouth Dissolving Tablet
Nippo 200 mg (Nimesulide 200 mg) Tablet is a non-selective nonsteroidal anti-inflammatory drug (NSAID) which is used as a second-line therapy to treat acute pain associated with osteoarthritis and menstruation. It is also used to treat mild to moderate pain caused due to sprains and strains of joints and muscles.
Uses of Nippo 200 Tablet (Nimesulide 200 mg)
- -Acute Pain
-This medicine is used to relieve pain and swelling of joints and muscles.
- -Primary Dysmenorrhea (Painful menstruation cycle)
-This medicine is used to relieve acute pain associated with menstruation.
- -Osteoarthritic Pain
-This medicine is used to relieve the symptomatic pain associated with Osteoarthritis
MECHANISM OF ACTION OF NIMESULIDE 200MG (NIPPO 200 MD TABLET) :
NIMESULIDE IS A NONSTEROIDAL ANTI-INFLAMMATORY DRUG (NSAID) WITH ANTI-INFLAMMATORY, ANTI-PYRETIC, AND ANALGESIC PROPERTIES. IT INHIBITS PROSTAGLANDIN SYNTHETASE/CYCLOOXYGENASE, WHICH LIMITS PROSTAGLANDIN PRODUCTION. ITS CYCLOOXYGENASE INHIBITING POTENCY IS INTERMEDIATE, BUT IS RELATIVELY SELECTIVE FOR THE CYCLO-OXYGENASE-2 (COX-2) THUS THE POTENTIAL FOR GASTRIC INJURY AND INTOLERANCE IS LESS. IT IS ALSO A FREE RADICAL SCAVENGER, AND HELPS PROTECT AGAINST THE TISSUE DAMAGE THAT OCCURS DURING INFLAMMATION. ABSORPTION: WELL ABSORBED FROM GI TRACT FOLLOWING ORAL ADMIN. PEAK PLASMA LEVELS:1-3 HR. WITH BID ADMIN OF 100 MG, STEADY-STATE IS ACHIEVED WITHIN 24-36 HR. DISTRIBUTION: 99% BOUND TO PLASMA PROTEIN. METABOLISM: HEPATIC BIOTRANSFORMATION; PRINCIPAL METABOLITE IS 4-HYDROXY-NIMESULIDE. EXCRETION: ELIMINATION HALF-LIFE: 2-5 HR. METABOLITES IN URINE: 80%, FECES: 20% OF THE ADMINISTERED DOSE.9% BOUND TO PLASMA PROTEIN.
SPECIAL PRECAUTIONS FOR NIMESULIDE 200MG (NIPPO 200 MD TABLET) :
HISTORY OF GI TRACT DISEASE, INFECTIONS, OEDEMA, HYPERTENSION, ELDERLY, LACTATION.
SIDE EFFECTS OF NIMESULIDE 200MG (NIPPO 200 MD TABLET) :
EPIGASTRIC DISCOMFORT, HEARTBURN OR ABDOMINAL CRAMPS, NAUSEA, VOMITING AND DIARRHOEA; SKIN RASH, PRURITUS, OEDEMA, HEADACHE, DIZZINESS, DROWSINESS; HYPERSENSITIVITY REACTIONS (E.G. BRONCHOSPASM, RHINITIS, ANGIOEDEMA URTICARIA); GI HAEMORRHAGE/PERFORATION; BULLOUS/EROSIVE STOMATITIS, PURPURA, THROMBOCYTOPENIA, TOXIC EPIDERMAL NECROLYSIS, HAEMATURIA, OLIGURIA, AND RENAL FAILURE; INCREASES IN LIVER ENZYMES. POTENTIALLY FATAL: FATAL HEPATITIS, STEVENS JOHNSON SYNDROME.
DRUG INTERACTIONS OF NIMESULIDE 200MG (NIPPO 200 MD TABLET) :
ADDITIVE HEPATOTOXIC EFFECTS WITH KNOWN HEPATOTOXINS: ANTI-CONVULSANTS (E.G. VALPROIC ACID), ANTI-FUNGALS (E.G. KETOCONAZOLE), ANTI-TUBERCULOUS DRUGS (E.G. ISONIAZID), TACRINE, PEMOLINE, AMIODARONE, METHOTREXATE, METHYLDOPA, AMOXICILLIN/CLAVULANIC ACID. MAY DECREASE THE ORAL BIOAVAILABILITY OF FUROSEMIDE AND THE NATRIURETIC AND DIURETIC RESPONSE TO FUROSEMIDE. INCREASED RISKS OF GI AND HEPATIC ADVERSE EFFECTS WITH OTHER NSAIDS, INCLUDING ASPIRIN. MAY INCREASE ANTI-COAGULANT EFFECT OF WARFARIN. POTENTIATES THE ACTION OF PHENYTOIN. MAY BE DISPLACED FROM BINDING SITES WITH FENOFIBRATE, SALICYLIC ACID, AND TOLBUTAMIDE. INTERACTIONS BETWEEN NSAIDS AND LITHIUM, PROBENECID AND CICLOSPORIN, HAVE BEEN DOCUMENTED.
CONTRAINDICATIONS OF NIMESULIDE 200MG(NIPPO 200 MD TABLET) :
HYPERSENSITIVITY; GI BLEEDING, ACTIVE PEPTIC ULCER DISEASE; SEVERE RENAL AND HEART FAILURE; HEPATIC IMPAIRMENT OR KNOWN LIVER DISEASE; COAGULATION DISORDERS; PREGNANCY; CHILDREN <12 YR.
FOR USE OF REGISTERED MEDICAL PRACTITIONER OR A HOSPITAL ONLY